As non-essential and elective medical procedures resume, the landscape of medical device manufacturing is undergoing a resurgence. The year 2022 stands as a pivotal time for industry advancements, addressing contemporary supply challenges and ushering in innovative, cutting-edge products. To navigate this terrain successfully, OEMs within the life science sector are embracing novel technologies to excel—embracing the full potential of metal injection molding (MIM).
MIM is swiftly gaining widespread adoption within the realm of medical device manufacturing. Serving as a cost-effective and scalable alternative to traditional CNC machining and manufacturing methods, MIM has the capability to craft intricate geometries previously deemed unattainable, all while upholding stringent quality standards. By harnessing MIM, OEMs can achieve enduring cost efficiencies throughout the production process and meet the most demanding timelines for research and development (R&D) and new product introductions (NPIs).
Whether OEMs are contemplating a transition for their existing product lines or aiming to introduce new offerings to the market, MIM effectively resolves challenges in medical device manufacturing.
Scalable Process, Cost-Effective Components, and Uncompromising Quality
MIM technology empowers OEMs to fulfill the increasing demand for advanced medical device technology, simultaneously achieving lasting cost efficiencies and consistently delivering components of superior quality that meet stringent material durability requirements. The metal injection molding process offers exceptional control over tolerances, particularly for high-density ferrous and bio-implantable alloys meticulously crafted through precise metallurgical methods. This precision is complemented by an unparalleled scalability that outperforms conventional machining and alternative manufacturing approaches.
Throughout the MIM process, powdered metal feedstock is precisely injected into molds and then sintered at elevated temperatures, yielding intricate, high-density, and high-performance parts in substantial quantities. This method of production streamlines operations, leading to enhanced efficiency and ultimately reducing the cost per unit.
Collectively, these benefits position MIM as a sustainable solution for expanding production capacity while upholding unwavering quality benchmarks—resulting in an overall improved value proposition. However, the potential of MIM extends beyond existing medical device manufacturing. Exploring MIM during the initial phases of product development grants OEMs a substantial advantage in new product introductions (NPIs) over their competitors.
The versatility of MIM provides medical device engineers with unmatched creative freedom.
Medical devices are constantly evolving, and quickly, which is no small task for manufacturers when dealing with complex, highly regulated components. OEMs need a solution to balance fast, efficient R&D that brings the best possible product to market with long-term supply sustainability and cost savings. Smart MIM strategies can allow life science OEMs to enjoy the best of both worlds.
Throughout the R&D process engineers and project managers are tasked with delivering the best possible iterations of components within shorter and shorter timelines. Enter MIM. Employing the attributes of metal injection molding during the design and development phases frees engineers from many of the inherent with other manufacturing methods and ensures they have a robust and scalable design featuring geometries formed by injection molding tooling. The result: better components on the market without lengthening timelines. Add the long-term cost and scalability benefits and MIM is a clear choice for med device OEMs.
Using strategies to speed up R&D, including 3D metal printing and rapid MIM tooling for prototyping and testing low-volume production during initial product validation, can allow OEMs to reap minimized costs and realized efficiencies. Manufacturing prototype components in under 48 hours, 3D metal powder printing doesn’t require tooling, so components are printed with MIM powders and tested to full functionality without the typical waiting time and cost. With increased geometry capabilities, this tool-free method meets MIM material standards and delivers metal components in days. Similarly, rapid-MIM tooling can design, build and deliver low-volume production representative components in as little as six weeks.
Regardless of the specific method, when used strategically, MIM is a sustainable solution that offers unmatched design freedom in the product validation and testing phases — allowing your products to hit the market faster and more efficiently. Combined with the overall benefits of metal injection molding for scalable, cost-effective production, MIM technology solves headaches and gives life science OEMs a competitive edge when bringing new products to market.
Gain insights into how the latest innovations in MIM technology are elevating the realm of medical device manufacturing to new heights.



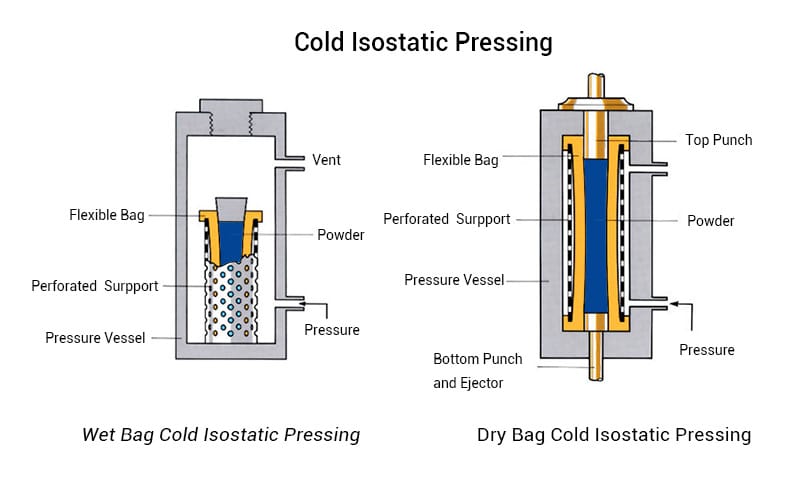





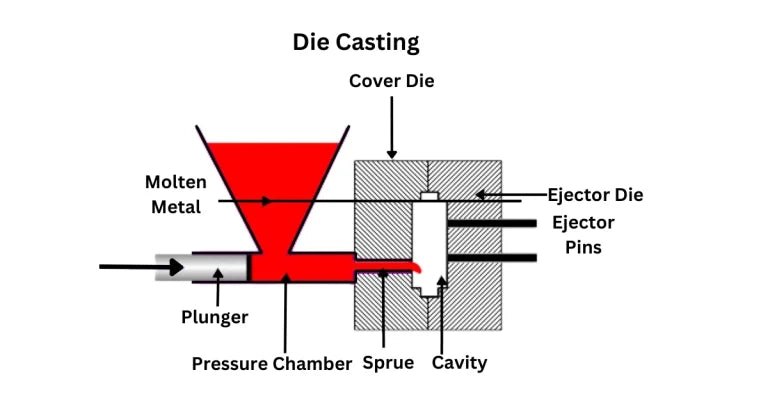



Share:
Vacuum Heat Treatment Process for Large and Complex Moulds
Requirements for Organic Adhesives in Metal Powder Injection Moulding Technology