Powder Metallurgy (PM) is an advanced material preparation technology that uses metal powder or a mixture of metal and non-metal powder as raw materials and manufactures metal materials, composite materials and various types of products through processes such as forming and sintering.

1、Important Advantages and Disadvantages of Powder Metallurgy Technology Important Advantages:
① It can prepare some materials that are difficult to prepare by other methods, such as refractory metals, pseudo alloys, porous materials, and special functional materials (hard alloys);
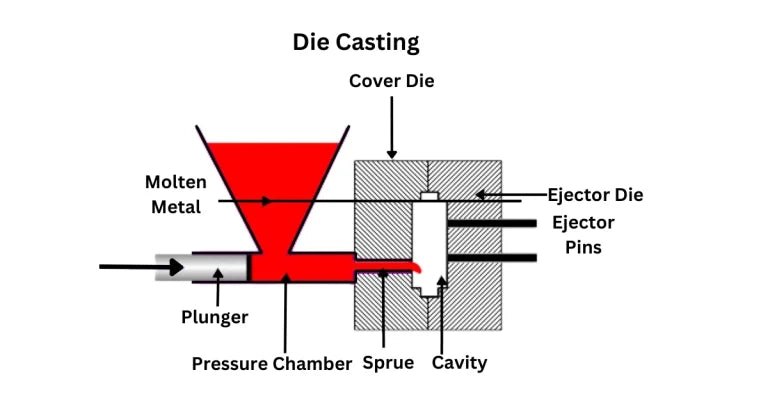
② Because powder metallurgy uses a mold that is very close to the shape of the final product during the forming process, the product processing volume is small and material is saved;
③ For some products, especially those with special shapes, it is easy to produce using molds, and the workpiece processing volume is small, and the production cost is low, such as gear products.
Important disadvantages:
① Since the pores in powder metallurgy products are difficult to eliminate, the mechanical properties of powder metallurgy products are lower than those of the same casting products;
② Since the forming process requires molds and corresponding presses, large workpieces or products are difficult to manufacture;
③ The scale effect is relatively small (Advantages: high material utilization, low processing cost, labor saving, and materials or products with special properties can be obtained. Disadvantages: Due to the presence of pores in the product, the material performance is poorer than that of traditional processing methods. Example: copper-tungsten pseudo alloy manufacturing, which is a material that cannot be obtained by traditional methods)
2、Analyze which stage in the powder metallurgy process improves the material utilization rate, and why?
In the powder metallurgy process, the material utilization rate is improved by the die pressing process. Because the mold design is close to the size of the final product, the pressed blank is often very close to the size of the product used, the material processing volume is small, and the utilization rate is high; for example, when producing automobile gears, if mechanical methods are used, the process is long and the material processing volume is large, while the powder metallurgy forming process can use the mold forming powder to obtain a shape and size close to the final product. Compared with the mechanical processing method, the processing volume is very small, saving a lot of material.

3、The gas atomization powder making process can be decomposed into several areas. What are the characteristics of each area?
The gas atomization powder making process can be decomposed into four areas: metal liquid flow negative pressure turbulence area, original droplet formation area, effective atomization area and cooling solidification area. Its characteristics are as follows: metal liquid flow turbulence area: under the reflux of the atomizing gas, the flow of the metal flow column is hindered, the laminar state is destroyed, and turbulence is generated; original droplet formation area: due to the flushing of the lower end atomizing gas, the turbulent metal liquid flow is stretched, the metal flow column is broken, and the strip-tubular original droplets are formed; effective atomization area: due to the high-speed atomizing gas carrying a large amount of kinetic energy, the impact on the formation of strip-tubular original droplets breaks them into tiny metal droplets; cooling area solidification area: at this time, the tiny droplets leave the effective atomization area, cool, and gradually spheroidize due to the surface tension.
4、Analyze why blue tungsten is used as the raw material for the reduction preparation of tungsten powder?
The main advantages of using blue tungsten as raw material to prepare tungsten powder are:
① It is possible to obtain primary particles with fine particle size, although the secondary particles are larger than the secondary particles of tungsten powder prepared using WO3 as raw material.
② Using blue tungsten as raw material, the secondary particles of blue tungsten are large (the primary particles are small), less volatile in H2, the chance of growth through gas phase migration is reduced, and the WO2 particles obtained are small; after obtaining WO2 by one-stage reduction, it is further reduced at high temperature in dry hydrogen, the particle growth is not obvious, and the yield is high.

5、Analyze the relationship between powder particle size, particle size distribution, powder morphology and bulk density.
The bulk density is the mass of powder per unit volume when the powder naturally fills the container under specified conditions. It is an important physical property of the powder and an important process parameter in the powder metallurgy process. The powder particle size, powder shape and morphology have a significant impact on the bulk density:
① The smaller the average particle size of the powder, the more complex the powder morphology, the larger the gaps between the powder particles and on the powder surface, and the smaller the bulk density;
② The smaller the average particle size of the powder, the more complex the powder morphology, the greater the friction resistance between the powder particles, the worse the fluidity, and the smaller the bulk density;
③ The smaller the powder mass (pore factor in the powder particles), the smaller the bulk density;
④ Adding a small amount of powder with a smaller particle size to some large-diameter powders to form a certain particle size distribution is conducive to improving the bulk density.
6、In the process of gas atomization powder making, what factors control the powder particle size?
The angle between the two streams is larger, the stronger the impact of the atomizing medium on the metal flow column, and the finer the powder obtained; when using liquid atomizing medium, because the mass is greater than that of gas atomizing medium, the energy carried is large, and the powder obtained is finer; the diameter of the metal flow column is small, and the powder particle size obtained is small; the higher the metal temperature, the smaller the viscosity of the metal melt, the easier it is to break, and the finer the powder obtained; the greater the medium pressure, the stronger the impact, and the finer the powder.
7、What is the basic principle of testing powder particle size using specific surface adsorption method?
Powder has an adsorption effect on gas due to its large total surface area and unbalanced surface atomic force field. In the liquid nitrogen temperature zone, the adsorption of gas by substances is mainly physical adsorption (no chemical reaction). After mathematical processing, if the total volume of adsorbed gas is known, it is converted into the number of gas molecules and divided by the volume of a gas molecule to obtain the surface area of the powder. Usually one gram of powder is used for measurement, so we define the surface area of one gram of powder as the specific surface area. When we know the total surface area value, we can assume that the powder is spherical, and then obtain the average particle size of the powder based on the relationship between the equivalent diameter of the sphere and the surface area (shape factor). In order to obtain accurate measurement data as much as possible, the adsorbed gas is usually an inert gas. This method of measuring the total surface area of a certain mass of powder and then calculating the average particle size of the powder is the basic principle of calculating the powder particle size by testing the specific surface area of the powder.
8、Analyze the differences and stress characteristics of uniaxial pressing and isostatic pressing respectively, and compare the differences between hot pressing and hot isostatic pressing.
The difference between uniaxial pressing and isostatic pressing lies in the different stress states of the powder. Generally, uniaxial pressing is completed in a rigid mold, while isostatic pressing is performed in a soft mold. During uniaxial pressing, since the external force is only applied in the uniaxial direction, the pressure on the mold wall side is less than the force in the pressing direction, so the stress state is anisotropic, σ 1 》σ 2= σ 3, resulting in uneven density distribution in various parts of the pressed green sheet. During isostatic pressing, since the stress comes evenly from all directions and is carried out through hydrostatic pressure, the pressure in each direction is equal, and the stress distribution in various parts of the powder is uniform, σ 1= σ 2= σ 3, so the density in various parts of the pressed green sheet is basically the same.

9、Analyze the principle of preparing tungsten powder by reduction and the factors affecting the growth of tungsten powder particles.
Tungsten powder is obtained by the process of reducing tungsten oxide powder with hydrogen. During the reduction process, the oxide changes from high valence to low valence, and finally is reduced to tungsten powder, WO3—WO2 — W; there are also oxide forms such as WO2. 90—WO2. 72. When the temperature is higher than 550 degrees, hydrogen can reduce WO3, and when the temperature is higher than 700 degrees, hydrogen can reduce WO2. Under this condition, the oxygen dissociation pressure of water molecules is lower than that of WO3 and WO2, and water molecules are relatively stable. WO3 and WO2 are reduced. At the same time, due to the effect of temperature, the reduction products in the loose powder are easily discharged by diffusion, and the reduction kinetic conditions are met, resulting in the reduction of tungsten oxide by hydrogen.
Since WO3 and WO2 have a large volatile pressure in hydrogen containing water molecules, and the higher the reduction temperature, the greater the volatile pressure, after the tungsten oxide entering the gas phase is reduced, it settles on the reduced tungsten powder particles, causing the tungsten powder particles to grow. The long time the powder stays in the high temperature zone will also cause the tungsten powder particles to grow due to atomic migration. The high humidity of hydrogen causes WO3 and WO2 fine particles to enter the gas phase, which is also an important factor leading to the growth of tungsten powder particles.










Share:
Preparation Method of Metal Powder
Principle of Metal Powder Preparation by Gas Atomization and Factors Affecting Powder Performance